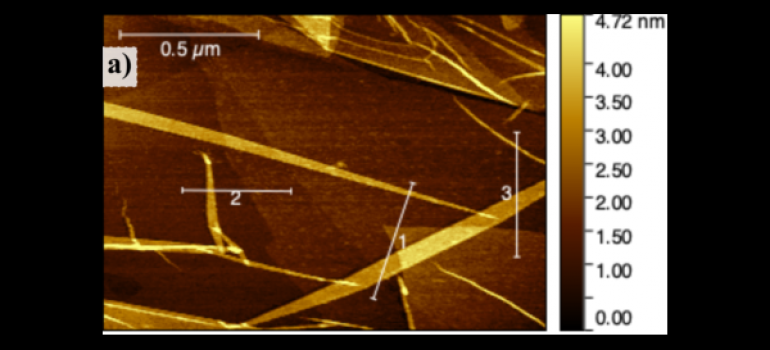
Electrochemical processes form the basis for many processes and devices: the purification of metals, metal deposition; the detection method used in some biosensors; the creation of electrical energy in a fuel cell or battery.
Characterization of the structure of the electrochemical interface, identifying and evaluating the kinetics of the processes occurring on the surface are important to understand, improve and design new electrochemical devices.
Our main research focus is the study of electrode surfaces and the processes occurring on them, from the dynamics of adsorbed molecular films, to the kinetics of redox reactions on nano particle surfaces in fuel cells.
Study of the electrode surface requires the use of many electrochemical techniques as well as in-situ spectroscopic and microscopic tools. A summary of the techniques used can be found here.
Making and Characterizing Modified Electrode Surfaces
We investigate how the electric potential changes the nature of the molecules on the electrode surface in addition to the arrangement of these molecules on the surface. We probe these changes using electrochemical methods (voltammetry, impedance, chronocoulometry) and couple them with in-situ spectroscopies such as FTIR, reflectance, Raman, and fluorescence in addition to AFM.
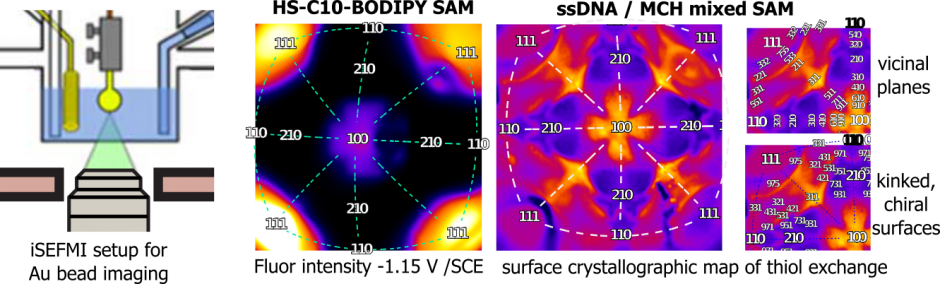
Imaging of a single crystal Au bead electrode, schematic, fluorophore modified alklythiol SAM, fluorophore modified ssDNA SAM.
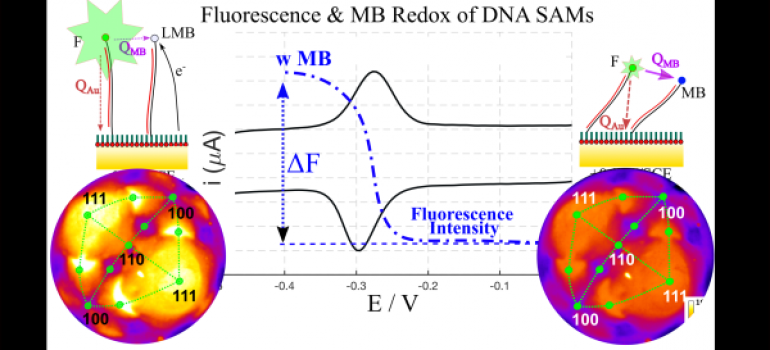
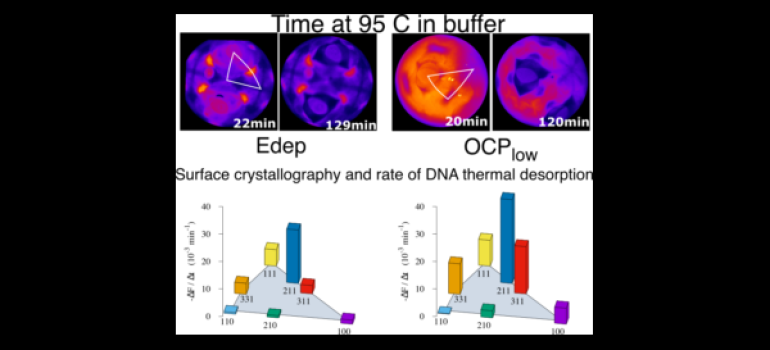
An example of an in-situ fluorescence image of a gold bead electrode surface modified with a DNA SAM labeled with a green emitting fluorophore is shown above (color represents the intensity). The underlying structure of the Au surface is clearly visible (the four eye shaped features are Au(111) facets). The figure below is a model of the single crystal bead surface with 111(red), 100(blue), 110 (green) facets highlighted.

model of a gold single crystal bead electrode
More about these studies can be found here.
Electrochemical DNA Sensors
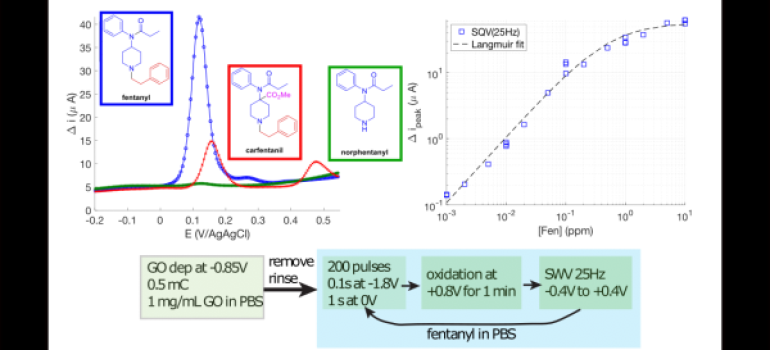
- Using new methods to deposit DNA SAMs on gold surface, our goal is to prepare electrochemical DNA based sensors for nucleic acid targets.
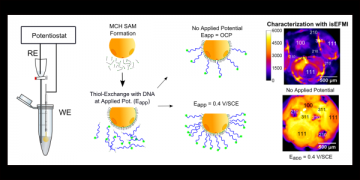
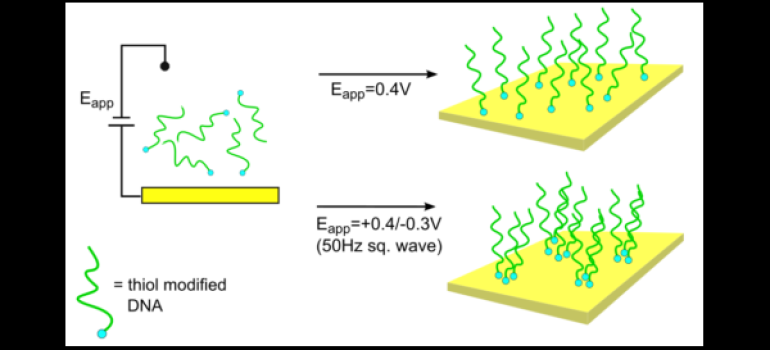
Electrodeposition, or potential assisted deposition of DNA SAM
- Decorating the gold surface with DNA nanocubes has been developed, characterized and demonstrated in a simple sensing method. (see https://doi.org/10.1021/jacs.4c09240)

DNA nanocubes on a gold electrode
- New methods for measuring hybridization isotherms are also being developed focussing on the coupling of fluorescence and electrochemical redox measurements
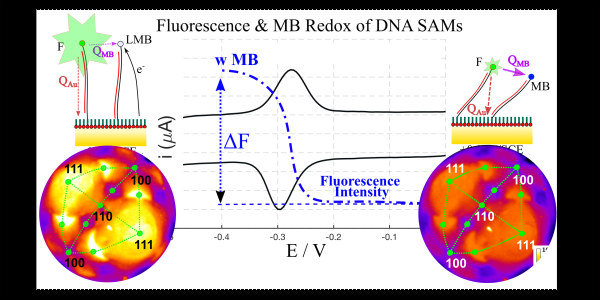
Using MB redox to manipulate the fluorescence intensity from a DNA SAM on a gold single crystal bead electrode
More about this work can be found here.
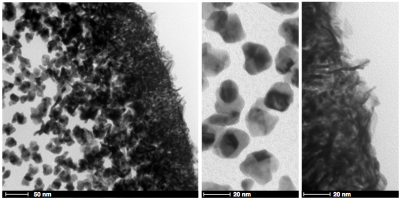
Nanoparticle Synthesis and Electrocatalysis
We also apply these techniques to probe the electrocatalytic properties of Pt and Pt – alloy catalysts that may find use in fuel cell applications. More about this work can be found here.
Prospective graduate students, please get in touch to learn more about research opportunities. For more information about the requirements for grad school, go to the Chem department’s grad student info page.
Honours students in Chemistry, interested in undergraduate thesis projects (Chem 449) should consider getting in contact during their third year (Chem 311 is highly recommended as well as Chem 403).
Interested PDFs should explore the possibilities for external funding provided by NSERC and UBC(Killam).